-
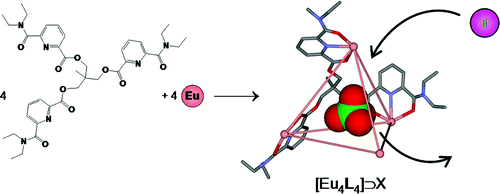
Lanthanide-mediated triangular cationic assemblies: structural and physico-chemical properties

S. Zebret, N. Dupont, C. Besnard, G. Bernardinelli and J. Hamacek
Dalton Transactions, 41 (16) (2012), p4817-4824


DOI:10.1039/c2dt12227h | unige:20257 | Abstract | Article HTML | Article PDF
This contribution investigates LnIII complexes formed with a small ditopic ligand, L1, and their structural, thermodynamic and photophysical properties. The spectrophotometric and NMR titrations evidence the triangular assemblies [Ln3(L1-H)3]6+ at stoichiometric conditions and their properties are discussed in relation to L2-containing analogues. In addition, the dinuclear species, [Ln2(L1-H)]5+, is observed with an excess of metal.